Financial Times: The Transformative Potential of Computerised Brain Implants

Clive Cookson in London and Richard Waters in San Diego
Seven years ago Michel Roccati swerved to avoid an animal while riding his motorbike near Turin and smashed into a roadside bench. The crash “exploded the bones in my back”, Roccati says, severing his spinal cord and cutting all communication between his brain and legs. “My doctors told me then that I would never be able to stand again, let alone walk,” says Roccati, who is 32. Then he activates a series of electrodes that neurosurgeons in Lausanne threaded along his spinal cord in a pioneering operation in 2020. His body shudders gently for a second or two and he rises from his chair, stepping out confidently across the room, though holding on to a walker for balance. “Every day my ability to walk improves, as my muscles become stronger and my nerves gradually regenerate,” he says. Roccati is one of a growing group of people benefiting from radically new forms of neurotechnology, under development at university and company labs in Europe and North America, which use computerised implants to interact with the human brain and central nervous system. These brain-computer interfaces can bypass neural impediments that prevent people who are severely disabled by accident or disease from moving their limbs — and enable those who cannot speak or operate a keyboard to communicate. Within a few years BCIs could develop into a market worth several billion dollars a year treating patients with severe motor impairment through injury or disease, according to Michael Mager, chief executive of Precision Neuroscience, a medical BCI company in the US.
The long-term implications are far greater. “We are creating a link between human intelligence and artificial intelligence,” says Mager. “It is possible that the only use for that fundamental connection will be paralysis, but I think it’s very unlikely.” Elon Musk founded Neuralink — the best known BCI company — in 2016 with a view to developing general purpose technology for connecting human brains and machines. Musk has long talked about using such links to essentially merge human intelligence with AI. For instance, he claims that greatly increasing the speed at which the brain can absorb and communicate information might overcome what he sees as one of the main limits to the ability of humans to keep up with advances in machine intelligence. But long before the cyborg future Musk dreams of, many in the field predict the technology will be used in more practical ways to overcome personal physical constraints and enhance individual performance, for instance by sharpening peoples’ visual and auditory powers, or boosting memory. “We are some way away from that but I don’t think it’s hard to imagine over time this technology being adopted by people who are otherwise healthy,” says Mager, who co-founded Precision in 2021 with Benjamin Rapoport, a founding member of Neuralink. The technical hurdles remain high. Collecting, exporting and interpreting signals from the brain is still a science in its infancy, while the invasive brain surgery required rules the procedure out for all but severely disabled patients.
At the same time, the technology raises profound ethical questions. “The medical side is already well protected by regulators and existing regulations,” says Rafael Yuste, director of Columbia University’s NeuroTechnology Center in New York. “But the technology will balloon and spread to the non-medical side,” he adds, posing significant new questions about how far people should be allowed to go to enhance their mental abilities, for example their memories. Yet in the lab, using brain signals to activate computers and other machines — something that until recently sounded like science fiction — is becoming almost routine, setting the technology on a path that could have long-term consequences. “This is a turning point for humanity,” says Yuste. “For the first time we have technology that can change the essence of who we are by getting into the brain, the organ that generates all of our mental and cognitive abilities.”
Neurosurgeons around the world have implanted electrodes into the human brain for decades as a treatment for Parkinson’s disease and other movement disorders, calming down the irregular electrical activity responsible for some symptoms. More than 160,000 patients have received “deep brain stimulation” of this sort. But the new wave of neural implants is far more sophisticated, allowing two-way communication between brain and device, says Jocelyne Bloch, a neurosurgeon at Lausanne University Hospital. She operated on Roccati and a subsequent paraplegic patient, Gert-Jan Oskam, who has two implants in his brain and spine communicating wirelessly via a “digital bridge”. The potential demand is huge. Beyond giving severely paralysed people the power to communicate and move again, medical uses could range from tackling sight and hearing loss to improving the treatment of chronic pain and psychiatric conditions by giving caregivers a precise, detailed picture of what is happening inside the brain.
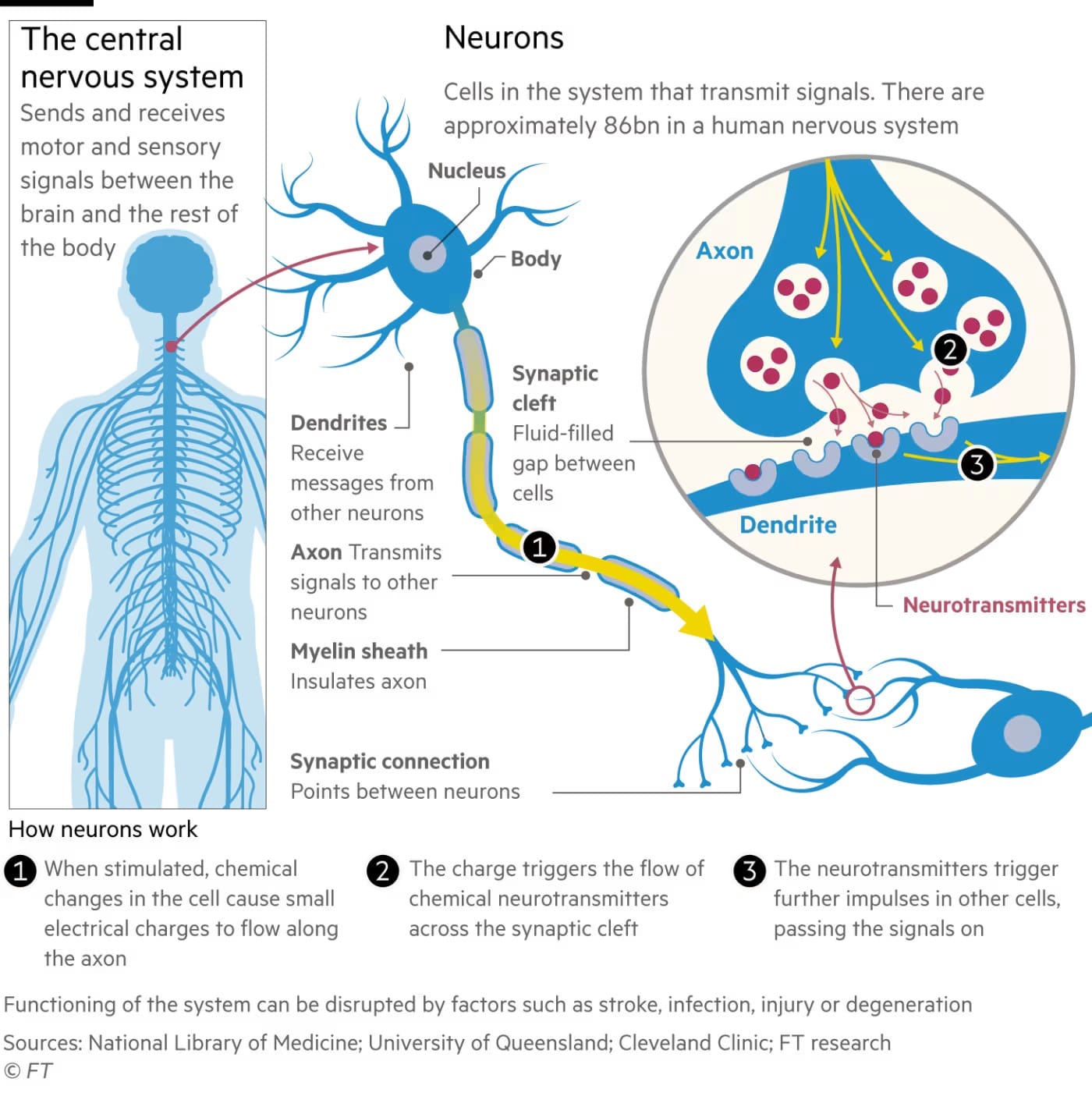
Yet few medical BCIs have been implanted into humans so far, as pioneering academic and corporate labs proceed slowly to prove their safety and efficacy. “We move cautiously, patient by patient,” says Henri Lorach who runs clinical trials with Bloch in Lausanne. Although external BCIs placed on the skin or scalp can detect and modulate neural activity to some extent, “the only way to record it sensitively and for extended periods is with a device placed surgically under the skull,” says Leigh Hochberg, director of the Center for Neurotechnology at Massachusetts General Hospital and of the long-running BrainGate BCI research programme. Only 50 or so patients worldwide have received a long-term prosthetic brain computer implant since clinical research started 20 years ago, Hochberg estimates. To accelerate clinical development, he and colleagues across the US set up the Implantable BCI Collaborative Community this year with the regulator, the Food and Drug Administration, joining as a key participant. “Because we learn a tremendous amount from each participant in these pilot clinical trials, I don’t think we’ll need large numbers taking part in the trials demonstrating safety and efficacy before applying for regulatory approval,” says Hochberg. “Maybe dozens of patients but certainly not the thousands who often take part in trials of new drugs.” Europe — Switzerland, in particular — has a significant presence in the BCI implant industry. Two companies, Onward Medical and Neurosoft Bioelectronics, are spinouts from École Polytechnique Fédérale de Lausanne (EPFL), the continent’s leading academic centre for neurotech research. A third, InBrain Neuroelectronics in Barcelona, is developing implants from graphene — sheets of carbon just one atom thick that was hailed as a wonder material after its discovery in 2004.
But experts on both sides of the Atlantic agree that American BCI companies have an advantage over their European counterparts. It is easier to fund and build an enduring company in the US, says Dave Marver, an American medtech leader who joined Onward in Lausanne as chief executive. “You have a larger pool of management talent in the US with more experience of commercialising things globally, more funding is available there and the regulatory regimes differ.” The FDA is better prepared to approve BCI clinical trials than its European counterparts, Marver says. When he came to Onward in 2020, he continues, “we didn’t have any clinical sites or plans to commercialise in Europe, because of the cost and complexity.” He quickly changed that policy. “I said: ‘We have paralysed people here, we are headquartered here and we’re going to commercialise here’.” Among the half dozen US companies testing BCI implants, “Elon Musk is clearly our biggest competitor,” says Grégoire Courtine, who heads EPFL’s neurotech research. But he insists that Onward, which has raised just over €170mn since its foundation in 2014, can match the performance of Musk’s Neuralink which has raised $687mn in funding, according to PitchBook. Neuralink is the most visible neurotech company in the media — and the least transparent. It communicates through occasional tweets and blog posts. After many animal tests, Neuralink’s first human subject, 30-year-old paraplegic Noland Arbaugh, received his Link implant at Barrow Neurological Institute in Arizona in January. In videos and blogs posted by Neuralink, Arbaugh enthused over the way the Link had enabled him to control a computer cursor. “It’s given me the ability to do things on my own again without needing my family at all hours of the day and night,” he said.
In the weeks after the surgery many of the 64 flexible recording leads that had been threaded through Arbaugh’s brain came loose. Neuralink engineers compensated by programming the device to be more sensitive to neural activity. The company is currently selecting a second volunteer to receive an improved version of the Link with electrodes that fit more securely in the brain — and will perhaps insert it as soon as this month. Competing neurotech companies vary considerably in the type of electrodes they implant into the brain. Researchers have most experience with the so-called Utah array, a rigid assembly of 96 electrodes that has been used for 20 years by the BrainGate academic consortium. The most recent BrainGate human study, published in Nature last August, placed two arrays each about the size of a small aspirin pill with a total of 128 electrodes into the cerebral cortex of Pat Bennett, 68, a patient with amyotrophic lateral sclerosis. She had lost the ability to talk. An AI algorithm decodes her neural activity, teaching itself to distinguish the patterns associated with her formulation of individual phonemes — the building blocks of spoken English. A language model then converts the stream of phonemes into a sequence of words that can be displayed on a computer screen or spoken by a synthetic voice at about 60 words per minute with reasonable accuracy. “Imagine how different conducting everyday activities like shopping, attending appointments, ordering food, going into a bank, talking on a phone, expressing love or appreciation — even arguing — will be when non-verbal people can communicate their thoughts in real time,” Bennett wrote at the time.
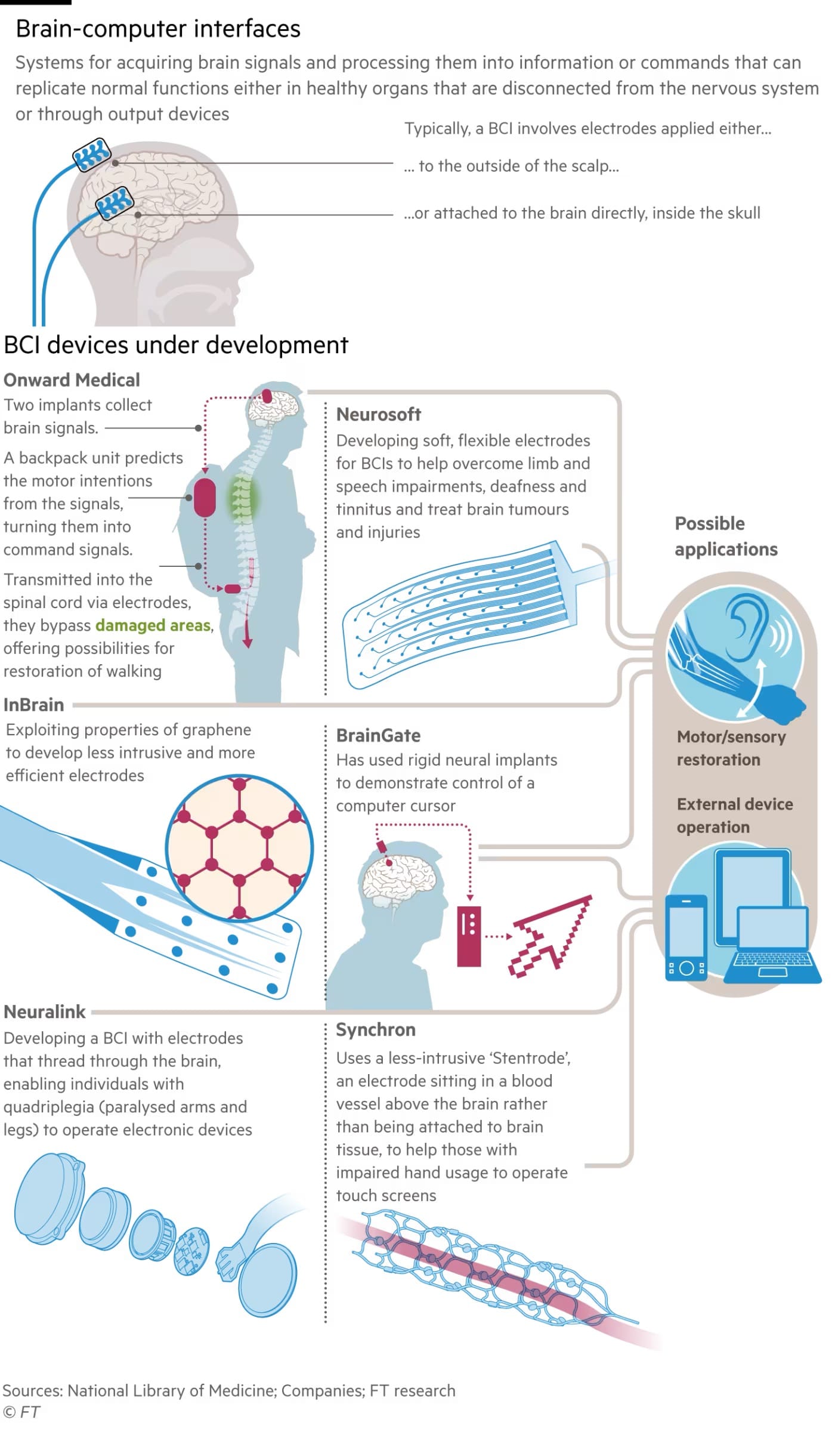
Other labs are developing more flexible and less invasive BCIs. Neurosoft Bioelectronics in Geneva is testing ultra-thin, soft electrodes deposited on silicone that wrap over the folds of the brain’s surface. Its technology has been tested successfully on three patients. InBrain’s graphene interface has similar selling points. It sits “on the cortex like a piece of cellophane without penetrating it,” says Carolina Aguilar, chief executive. Its first human trial is imminent. She sees Parkinson’s disease as InBrain’s first target, aiming to displace the “low density, low resolution” deep brain stimulation devices offered by medtech giants Boston Scientific and Medtronic. Some BCI companies are focusing on medical applications beyond disabled patients. Nicolas Vachicouras, Neurosoft chief executive, estimates that 60mn people worldwide have severe tinnitus, resulting in “severe depression and even suicide attempts, with no effective treatment available today”. The company’s research suggests that the cause is abnormal activity in the auditory cortex, giving the illusion of disturbing noise, which can be corrected with neuromodulation.
Among the US companies, Synchron stands a good chance to be the first to get an BCI implant to market. Much like a stent, its device is inserted into a blood vessel and sits above the motor cortex, the part of the brain that controls movement. It has been tested in 10 patients so far and publication of final results is expected soon, followed by a larger clinical study which the company hopes will lead to FDA approval. Although the device is not as sensitive to neural activity as some competitors, Synchron chief executive Tom Oxley says the company can achieve marketing approval more quickly with technology that does not require open brain surgery. “How can this technology improve people’s ability to live independently? That’ll be the marker of success,” he says. None of the implants are yet close enough to market for their cost to be an immediate issue but executives say pricing will have to reflect the huge potential benefits the technology offers patients with severe neurological disorders whose healthcare is very expensive.
“The key to creating a viable BCI industry, as well as ensuring patient access, is insurance coverage of the systems at a rate that is commensurate with the amount of money that these cost both to develop and to deliver,” says Precision’s Mager. “This needs to be a six-figure reimbursement.” Matt Angle, chief executive of Paradromics, a company in Texas developing a BCI with more electrodes than a standard implant, predicts that the first devices will cost “upwards of $100,000”, but “we would like to get to a place where brain-computer interfaces are costing on the order of what deep brain stimulators are costing today — let’s say $30,000.” Looking further ahead, Angle predicts that BCIs’ medical applications alone will create a market worth hundreds of billions of dollars — though many other experts in the field say it is far too soon to tell. “There is the potential to develop a dozen companies that are each worth more than $1bn in this space,” he says — and that is before BCIs are applied to enhancing human performance in areas from eyesight to memory. While such breakthroughs will depend on continued improvements in sensors and the microelectronics needed to capture and transmit brain signals, much also rests on broader advances in computing and AI. These include using cloud computing and applying AI to help interpret brain signals. Clinical trials today process data locally, says Courtine of EPFL, “but we do intend eventually to have all this brain information in the cloud, so we can train a large language model and create a brain GPT. Then we can learn from hours and hours of brain activity from a lot of people.”
Such projects are intensifying calls by ethicists to anticipate threats from misuse of neural data gathered from BCIs. Unesco, the UN scientific and cultural organisation, has convened a panel of 24 experts to draft recommendations on the ethics of neurotechnology, leading to a document for adoption by member states next year. Gabriela Ramos, who leads Unesco’s neurotech initiative, says: “We aim to ensure that these scientific and technological developments are aligned with our human rights.” That means more than protecting the privacy of people’s thoughts revealed by BCIs, she adds. For example, future implants may change an individual’s personality — for better or for worse — more extensively than existing brain treatments. Yuste at Columbia University, a vocal advocate for “neuro-rights”, points out that a few jurisdictions such as Chile and the US state of Colorado have already passed legislation to protect individuals’ neural data from exploitation. But his main concerns revolve around the non-medical uses of the technology, like enhancing mental performance. One way to protect people would be “to regulate the new consumer BCIs as if they were medical devices”, he says. For pioneering patients such as Roccati, such concerns are academic compared with the benefits that neurotechnology can bring. “My implant has already transformed my life,” he says, “and I’m looking forward to taking advantage of future advances in neurotechnology. So too are thousands of other people who can’t communicate or move their limbs but will be able to in future.”